About Us
The Food Safety and Drug Control Commissionerate (Drug Control Wing) is a Regulatory Agency under the Medical & Health Department of Rajasthan. It regulates the manufacture of drugs, Meedical Devices & cosmetics and sales of drugs in the state of Rajasthan. The Commissionerate is the head of the Commissionerate and for the enforcement of various Drug Laws, officers including Drug Controller, Assistant Drugs Controllers and Drugs Control Officers are posted at Head Quarters as well as in the field in all the districts.
The mission of the Food Safety and Drug Control Commissionerate (Drug Control Wing) is to protect the public health and to strive for the pharmaceutical excellence by ensuring the availability of safe, effective and quality drugs.
Food Safety and Drug Control Commissionerate (Drug Control Wing) functions for implementation and enforcement of Central Acts, namely
-- Drugs & Cosmetics Act 1940 & Rules 1945
-- Drugs Magic Remedies (Objec. Advert.) Act 1954
-- Drugs (Price Control) Order 2013
-- Medical Device Rules 2017
-- Cosmetic Rules 2020
The Commissionerate has developed and grown gradually; at present there are 116 posts of Drugs Control Officers sanctioned for different districts and 37 Assistant Drugs Controllers, besides two Drugs Controllers.
The techno – legal nature of duties and responsibilities of the regulatory officers constitute wide spectrum of activities within the State and coordinating some important actions with the Central Drugs Standard Control Organisation, New Delhi which are enumerated below :-
1. Grant of Manufacturing and Sales licences for Allopathic Drugs (Modern Medicine), Homoeopathic medicines through inspection.
2. Grant of Manufacturing licences of Cosmetics.
3. Grant and renewal of licences for operation of Blood Centres & grant of approval of Blood Storage Units.
4. Grant and retention of Medical device (Class 'A' & 'B') manufacturing licences.
5. Approval of Commercial Testing Laboratory for testing and analysis of Drugs and Cosmetics.
6. Monitoring and issuance Good Manufacturing Practices(GMP) Certificate, Good Laboratory Practices (GLP) Certificate, Market Standing Certificate (MSC), Non Conviction Certificate, Certificate of Pharmaceutical Products (COPP), Free Sale Certificate, WHO GMP Certificate etc.
7. Monitoring of quality of medicines and cosmetics through routine and statutory sampling, post marketing surveillance and recall of Not of Standard Quality (NSQ) medicines and cosmetics from the market.
8. Monitoring of the availability of life saving essential medicines and overcharging of medicines.
9. Detection of Spurious, Adulterated and Misbranded Drugs, Cosmetics & Medical Devices and launching prosecution against the offenders.
10. Investigation of complaints.
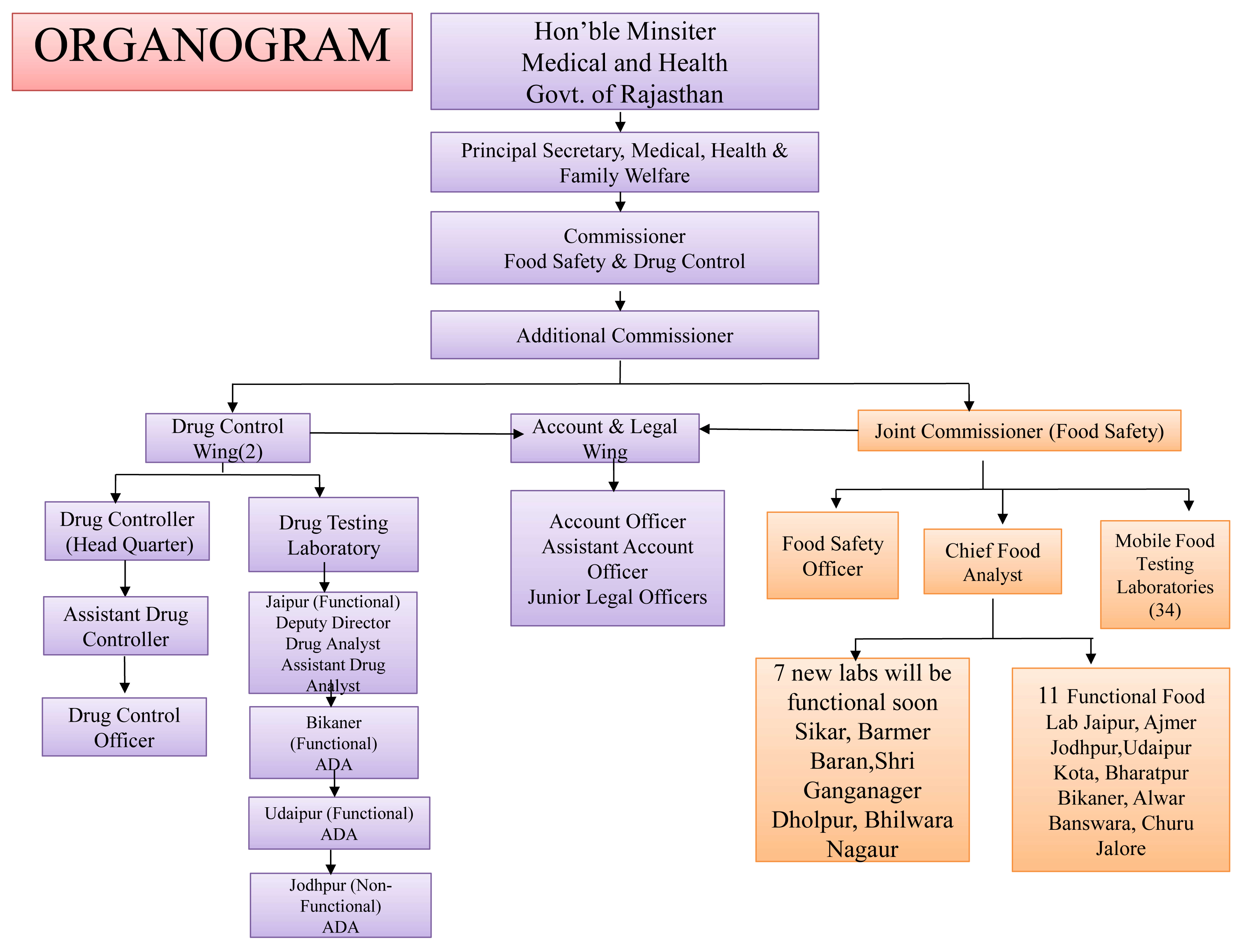
Organisational Chart
Services
The Drugs Control Organization, Rajasthan is a Regulatory Department. It regulates manufacture of drugs & cosmetics for sale, and sales of drugs. The Organization carries out following functions, which have interface with public.
Licensing – The Drugs Control Organisation grants following licenses:
-- Sale of drugs (medicines)
-- Manufacturing of drugs – Allopathic and Homoeopathic drugs
-- Manufacturing of Cosmetics
-- Manufacturing of Medical Devices
-- Operation of Blood Centres
Monitoring the Quality of Drugs and Cosmetics – This is achieved through constant vigil exercised by the field staff who conduct inspection of sales and manufacturing premises and carry out sampling of drugs and cosmetics. The samples of drugs and cosmetics are tested in the Govt Testing Laboratories.
Complaint Redressal – The complaints received from the public regarding quality of drugs and cosmetics or any other violation of Drugs and Cosmetics Act and Rules are attended and duly investigated with promptness and necessary action is taken in the matter.
Ensuring Availabilityof Drugs at proper prices – The field staff keeps an eye over the availability of drugs and the prices at which these are sold in the market, in order to ensure that these are not sold at prices higher than that allowed. The offenders are booked under law.
 चिकित्सा, स्वास्थ्य एवं परिवार कल्याण विभाग
Department of Medical, Health & Family Welfare
चिकित्सा, स्वास्थ्य एवं परिवार कल्याण विभाग
Department of Medical, Health & Family Welfare


